The main polluants affecting our health are sulfur dioxide (SO2), nitrogen oxides (NOx) and particulates. These contaminants may be directly emitted into the atmosphere (primary pollutants) or formed from primary pollutants that react in the atmosphere (secondary pollutants).
Examples of the latter are tropospheric ozone, which is formed in the presence of sunlight from volatile organic compounds (VOC) and nitrogen oxides (NOx); nitrogen dioxide, whis is formed by the oxidation of NO; and acid rain, which is formed when sulfur dioxide or nitrogen oxides react with water.
These pollutants have negative effects on human health (eye, nose, throat and lung irritation at low concentrations) and the environment, as they contribute to acidification and eutrophication, as well as the subsequent formation of particulates and tropospheric ozone (photochemical smog).
SO2 and NOx are mainly produced from the combustion of fossil fuel in high-temperature industrial processes and electricity generation.
Particulates are related to any type of industrial combustion and heating. Particulates and NOx are also related to traffic and transportation in general.
There are air quality surveillance booths throughout Spain measuring the concentration of these parameters. This information is available to the public and is used to determine if pollution levels in any particular area exceed the maximum permissible levels for health and the environment.
One of the consequences of air pollution is environmental acidification, which changes the chemical composition of soil and water and reduces their neutralising capacity. This is due to acid rain, i.e. acid precipitation on soil, caused by the emission of polluting gases into the atmosphere.

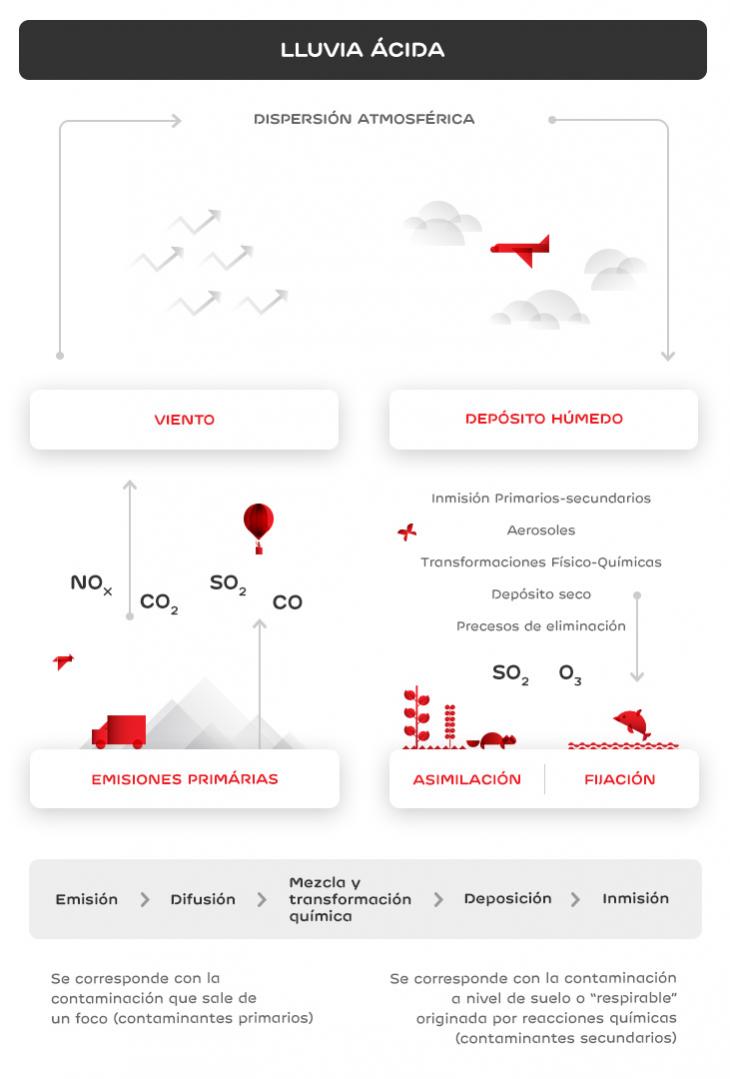
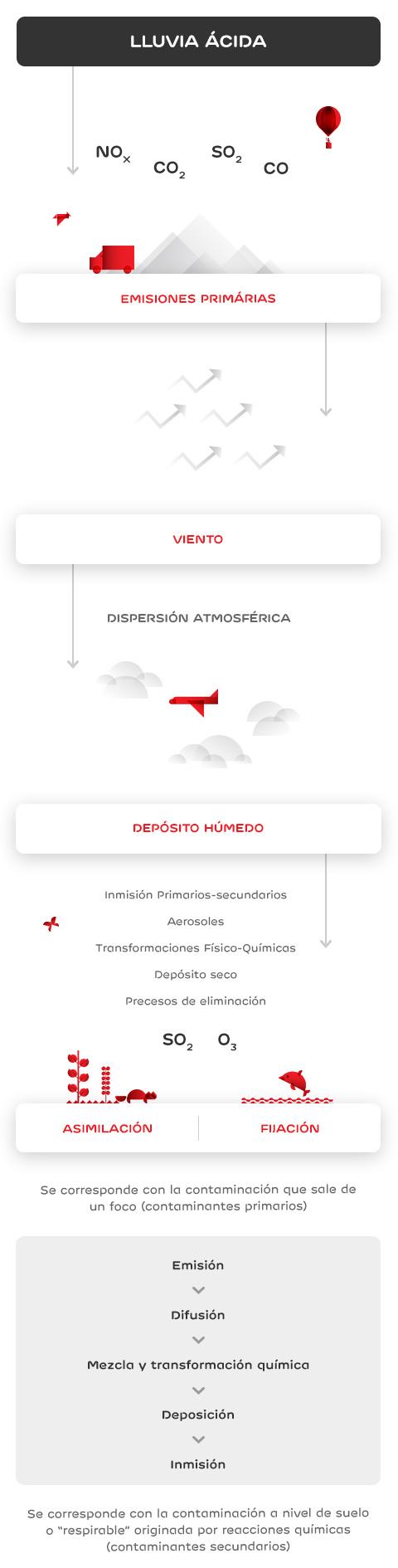
Acids, in the form of rain, snow or even fog, reach the surface of the planet, affecting aquifers, vegetation, wildlife and human populations.
Acid rain is corrosive, damaging the objects' paint and surface. It also damages marble or limestone buildings and monuments.
Acidification affects lakes, rivers and oceans, because it changes soil composition and pushes heavy metals into underground waters, thereby increasing their toxicity, preventing their consumption, and affecting the development of aquatic fauna.
The movement of soil aluminum and heavy metals prevents vegetation from absorbing water and nutrients properly. This gradually weakens trees and plants, making them more vulnerable to pests.
Polluting gases released into the atmosphere affect the respiratory system, causing diseases such as asthma and chronic bronchitis.
Photochemical smog (smoke + fog) is a dark, reddish-brown mist produced by environmental pollution and favored by thermal inversion. In times of high pressure, the lower layers of the atmosphere become much colder than the upper layers, and in the absence of air to promote mixing, the bottom layer is trapped, retaining stagnant air pollution. In the presence of sunlight, NOx and VOC agents, mainly caused by carbon-burning traffic and industrial processes, produce tropospheric ozone and secondary particulates that give rise to this phenomenon.
Smog is harmful to health because it irritates the eyes and the respiratory system (nose, throat), and it also aggravates allergies. Furthermore, smog produces climate changes because of the absence of rain, and without rain (or wind) nature cannot combat smog.

The formation of tropospheric ozone, which is harmful to health, should not be confused with the formation of stratospheric ozone, which is beneficial for the ozone layer and crucial for human life. While tropospheric ozone reduction is positive, the depletion of stratospheric ozone would cause greater solar radiation (mainly UV-B) and, therefore, global warming, which would further increase the risk of skin cancer, cataracts or premature skin aging, while also affecting vegetation growth and the aquatic ecosystem. The ban on Ozone-Depleting Substances (ODS) has successfully reduced the destruction of the stratospheric ozone layer.
As far as anthropogenic factors are concerned, the emission of gases containing chlorine and bromine (halogen source gases) is the cause for the depletion of the ozone layer. These gases accumulate in the lower atmosphere and are transported by wind and other air motions.
Once in the stratosphere, due to ultraviolet radiation, these gases quickly undergo chemical reactions and become reactive halogen gases - which, in turn, react with ozone and destroy it (depletion of the ozone layer). Over time, air in the stratosphere returns to the troposphere, bringing along reactive halogen gases which are then deposited on the Earth’s surface by rain and other precipitation. Some industrial processes and consumer products release halogenated substances containing chlorine and bromine atoms, which deplete the ozone layer. In turn, fluorine and iodine, although they are halogen atoms, either remain in chemical forms that do not destroy ozone (such as fluorine) or are, for the most part, eliminated in the atmosphere by natural processes and never reach the stratosphere.
The formation of the ozone hole requires abundant reactive halogen gases, temperatures low enough to form ice clouds or polar stratospheric clouds, isolation of air from other stratospheric regions, and sunlight.
With chlorine: Chlorofluorocarbons (CFC) and hydrochlorofluorocarbons or HCFC (transiently used to replace CFCs), carbon tetrachloride (CCI4) and methyl chloroform (CH3CCIC3; used in refrigeration, air conditioning, foams, aerosol propellants, metal cleaners and electronic components).
With bromine: Halons (Halon-1211, Halon-1301); used to extinguish fire and protect large computers, military equipment and spacecraft engines.
Methyl chloride (CH3CI) and methyl bromide (CH3Br): released by the terrestrial and oceanic ecosystem.
Infographics
Ozone layer and climate change
There is a connection between ozone depletion and climate change.

What measures are being taken to minimize the impact of these polluants?
The latest report on the quality of air in Spain (2015) showed that there had been no sulfur dioxide (SO2) exceedances. The reduction of this pollutant was partiularly signficant from 2008 onwards, due to investments in desulfurization plants for the electricity sector.
As for nitrogen oxides (NOx), the city of Madrid breached the hourly limit value (exceeding the hourly value of 200 µg/m3 by more than 18 times), while annual levels (40 µg/m3) were exceed in 5 areas: Madrid, Barcelona, Llobregat, Murcia and L'Horta (Valencia).
Traffic is the main source of nitrogen oxides, and for this reason legal norms - the most recent one was adopted in late 2015 (EURO 6) - are increasingly strict. Diesel cars produce high levels of NOx emissions, hence the implementation of higher parking fees for these cars in cities like London; it is also said that in 2020 diesel cars will be banned in cities such as Paris or even Madrid. Conversely, gasoline vehicles release more CO2 emissions, which - although not harmful to air quality - contribute to global warming.
As for emissions from fixed sources, the adoption of the new Industrial Emissions Directive determines stricter emission limits and, concomintantly, new investments such as denitrification stations for thermal power plants.
Spain has always had high levels of particulates, the concentration of which increases naturally with African dust intrusions.
In recent years, particulate concentration levels have been reduced from 10 exceedances in 2011 to 3 exceedances in 2014 (Tierras del Ebro, La Coruña and Central Asturias, exceeding the daily limit of 50 µg/m3 by more than 35 times), and only Central Asturias
exceeds the annual limit (40 ug/m3).
Due to the exceedances registered in the Principality of Asturias, the Regional Administration has prepared Air Quality improvement plans in Avilés and Gijón, as well as Action Protocols involving measures such as traffic restriction and public awareness initiatives,
in addition to specific actions for industrial activities in the area. Thus, the Aboño thermal plant must reduce its emissions if the Alert level is triggered due to adverse weather conditions.
For its part, EDP is conducting studies in collaboration with several university departments to determine the influence of the plant’s operations on the area, particularly in terms of air quality.
Some of the measures implemented by EDP’s thermal plants to minimize particulate emissions include the installation of electrostatic precipitators (performances above 90%). Desulphurisation plants, in addition to reducing sulfur oxides (performances above 95%), retain 50% of the particulates that manage to escape electrostatic precipitators.
These measures are complemented by other initiatives, already implemented by EDP in its environmental strategy and commitment, to reduce the company’s impact on the environment:
Building new electricity generation groups based on state-of-the-art technologies: combined cycles, and shutting down thermal carbon groups (Soto 1 in 2008 and Soto 2 in 2015).
Using waste gases from ArcelorMittal (blast furnace gases and coke battery gases) as fuel at the Aboño thermal station and the Sidergás cogeneration plant, enabling the recovery of these gases and avoiding the direct emission of more than one million tonnes of CO2 by flaring.
Starting the cogeneration plant for the Tudela Veguín cement factory in 2011, aimed at optimizing the energy consumption of this facility (which is used for grinding and drying the steel slags that are used in the production of high-strength cement). This has allowed the replacement of old oil-fired boilers with greater environmental impact.
Investments in renewable energy, which materialize through EDPR. By the end of 2015, this company had more than 2,100 MW of wind power installed in Spain.